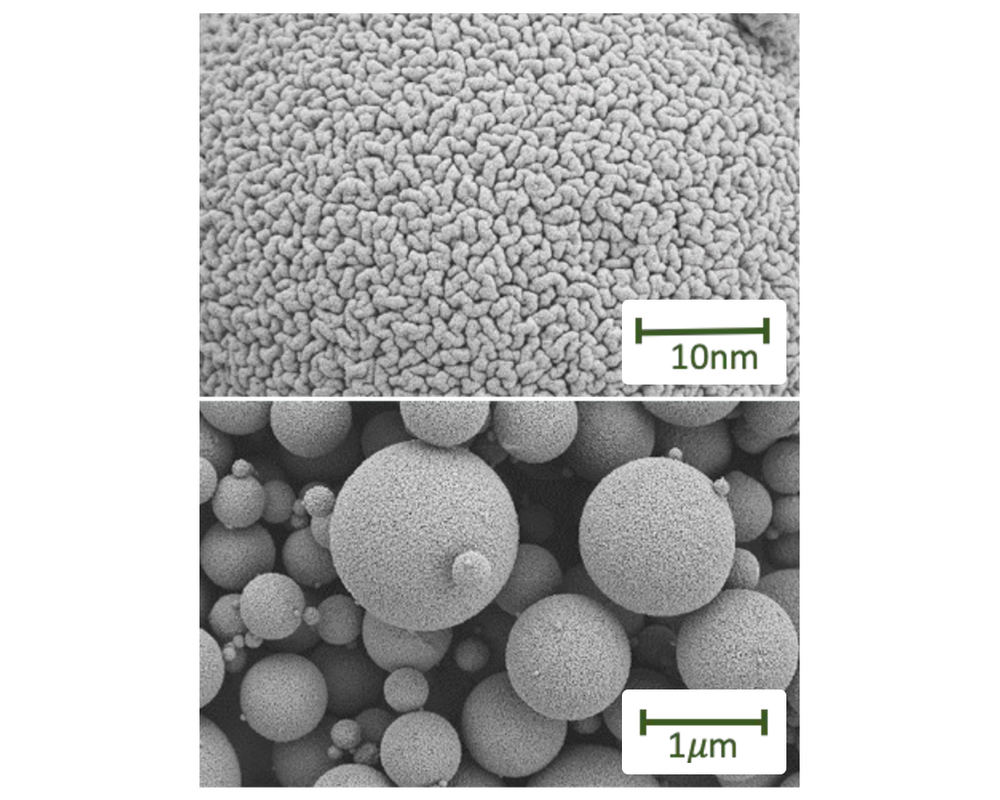
When administered as bare compounds, monoclonal antibodies (mAbs) and non-mAb proteins are unable to survive the acidic environment of the stomach and the enzymatic environment in the intestinal tract. Bioralix addresses this challenge by utilizing supercritical CO2 spray drying to encapsulate proteins into nanoparticles.
These nanoparticles are delivered to the intestine via enteric-coated capsules, which protect them through the stomach.
Upon release in the duodenum, the proteins cross the intestine wall and become available for pharmacological activity.
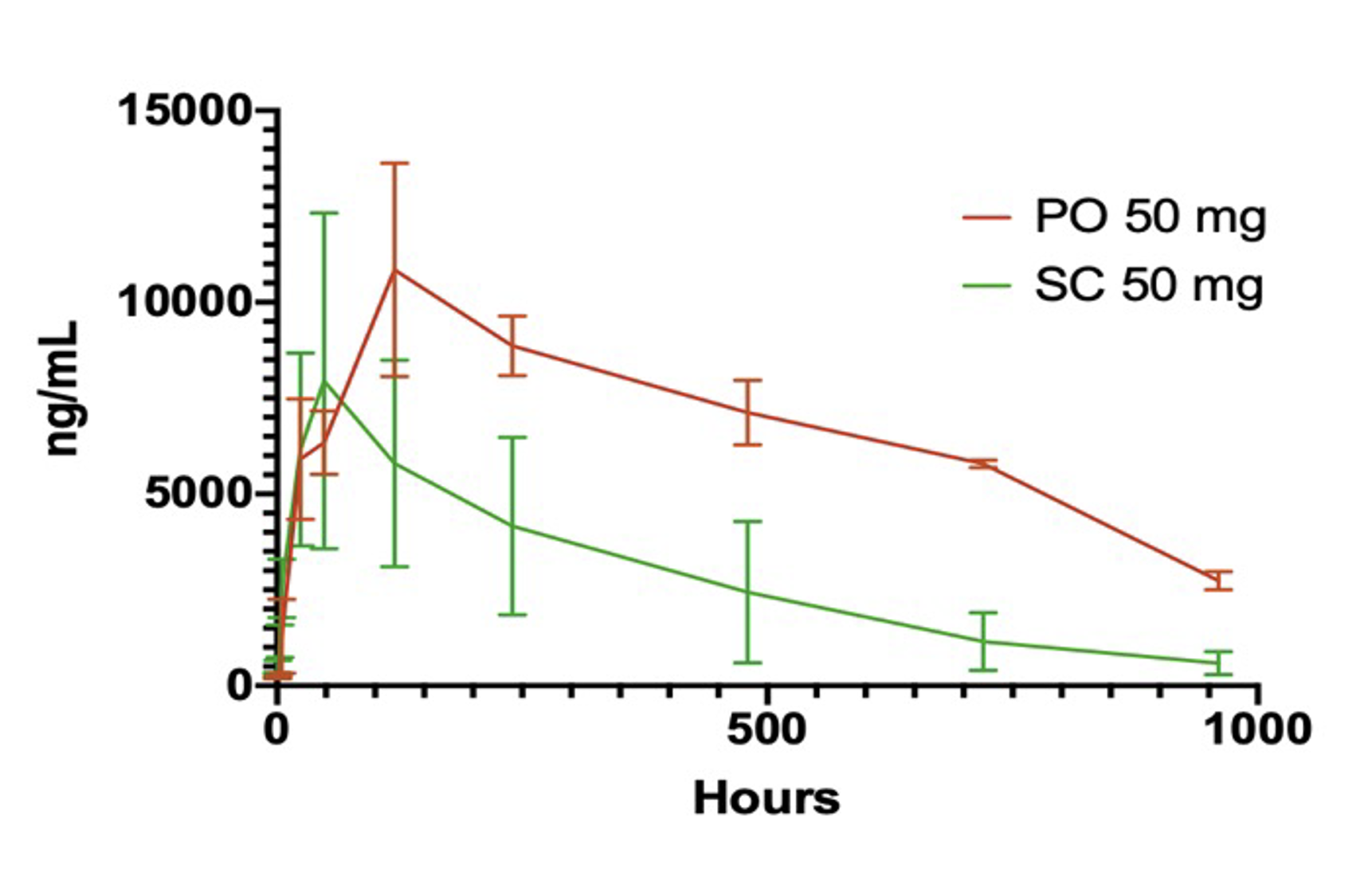
In three consecutive preclinical studies of increasing complexity, Bioralix demonstrated that the bioavailability of this oral delivery technology (PO), using omalizumab as a test compound, was over twice that of subcutaneous (SC) administration. In vivo proof-of-concept (PoC) repeat experiments are ongoing to validate these initial findings.
The current PoC study aims to demonstrate that:
• structure, binding affinity, and functionality of the proteins remain intact throughout the nanoparticle manufacturing and delivery process
• reproducible nanoparticle production (mAb load, particle size distribution) with superior stability can be achieved and most important, bioavailability is consistently higher than that of subcutaneous administration.