Field Lab
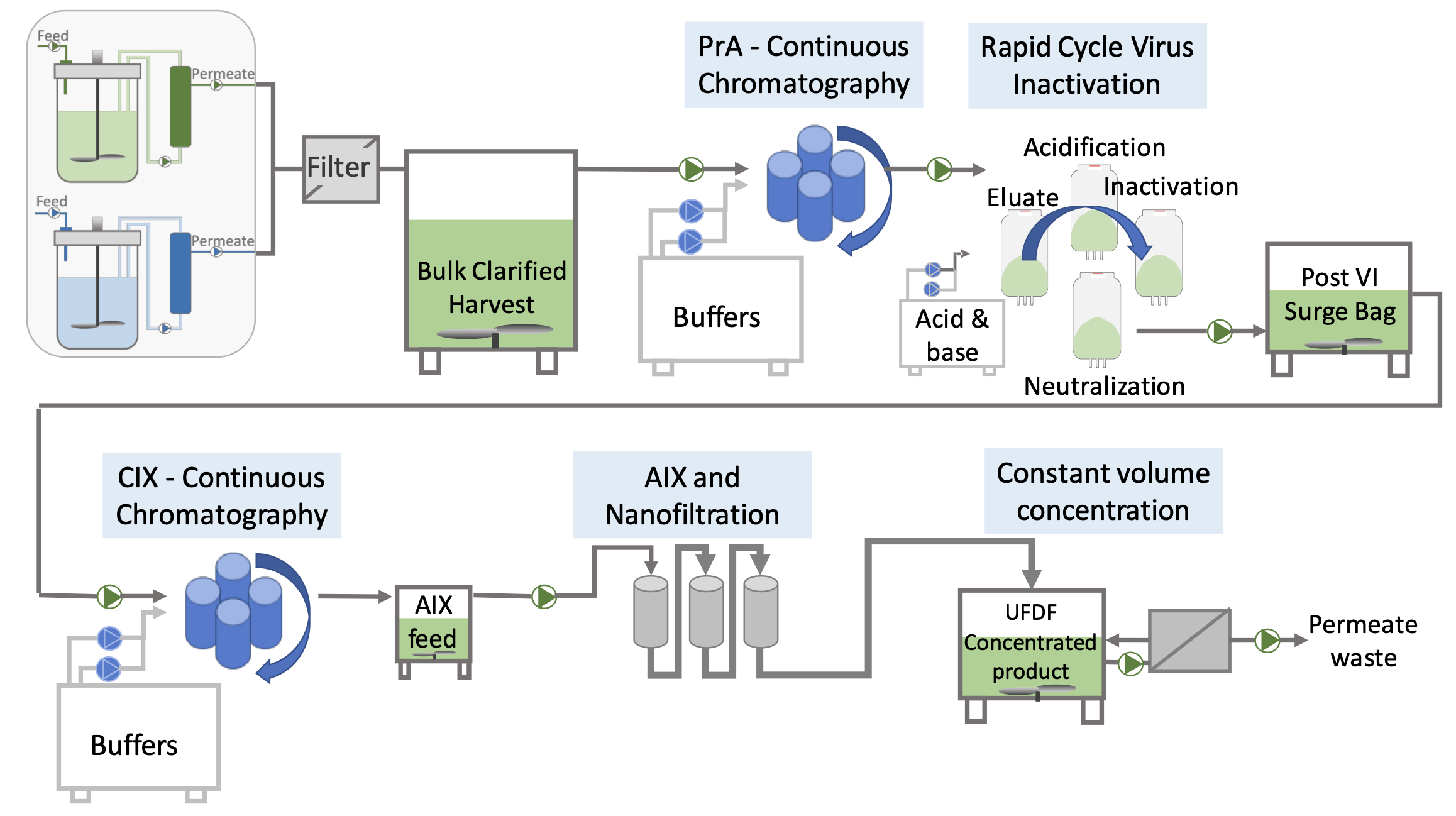
In our Field Lab, we set up and test your continuous process at the desired production scale, while simultaneously training all involved personnel. The operation of the 3C automation platform is thoroughly tested with all unit operations (chromatography and filtration systems) integrated, along with automatic sampling and analytical testing options.
Simulations of unit operations will be used to pretest purification performance. Once all systems function together as intended, the continuous manufacturing (CM) train is transferred to your biomanufacturing facility for installation and commissioning, overseen by BiosanaPharma. We also provide all necessary validation documentation templates as part of the CM DSP package to comply with the GMP legislations and guidelines.
Biodigital 3C Platform
Our biodigital 3C automation platform is part of an ongoing improvement program, where we continuously work on next generation equipment integration modules, system health dashboards, statistical data analysis, Process Analytical Technology (PAT), recipe optimization, and electronic (production) records.
These upgrade activities are further supported by Artificial Intelligence (AI) and Machine Learning (ML) technologies. Clients will have access to 3C platform upgrades every 1-2 years, with the latest version included for new 3C process implementations.